Ensuring Biosafety to protect Biodiversity in Sri Lanka


Project Manager – National Biosafety Project
Food and Agriculture Organization of the United Nations
If you drive few kilometers on roads in Sri Lanka outside of city limits, you are going to see many types of trees, plants and birds. This is merely a glimpse of biodiversity that is vastly spread across the entire country. Due to the perfect positioning on the globe, rich soil, varied topography and diverse climate, Sri Lanka possesses a wealth of biological diversity. Due to this gift from nature, the country is classified as one of the global “biodiversity hot spots” based on its hosting a large number of endemic plants and vertebrates.
Rich biodiversity is definitely a unique attraction for tourists who visit Sri Lanka. From archeological sites to scenic beauty, Sri Lanka has many to offer. The variety of flora and fauna that is found nowhere in the world, but in Sri Lanka has amazed all those who have visited this beautiful country. Therefore, protecting biodiversity is a prime responsibility of everyone including the authorities in the country.
The country has adopted a proactive approach towards formulating an environmental policy and has been one of the first countries to ratify the Convention on Biological Diversity (CBD) in 1994. The CBD is an international agreement designed to promote the conservation of biodiversity and to ensure the sustainable use and equitable sharing of genetic resources. The Ministry of Environment (MoE) acts as the national focal point for the CBD in Sri Lanka while the Biodiversity Secretariat (BDS) coming under its purview acts as the implementing center of actions.
Out of many threats to biodiversity, the potential adverse effects from genetically modified organisms (GMOs) have been a concern when it comes to their existence alongside non-GMOs. Therefore, many countries came to an agreement to ensure the safe handling, transport and use of GMOs resulting from modern biotechnology that may have adverse effects on biological diversity, taking also into account the risks to human health. This agreement known as the Cartagena Protocol on Biosafety (CPB) to the CBD entered into force in 2003.
The products resulting from modern biotechnology are increasingly being used in many countries to overcome the challenges in agriculture that the conventional methods have not addressed efficiently. Therefore, this marvelous technology cannot be ignored, especially when it comes to sustainable agriculture in the world and to reach the Sustainable Development Goal #2: Zero hunger.
Since modern biotechnology is a new technology, the products of it must be assessed for safety before they are utilized to solve the myriad of challenges, which threaten the welfare of humans. Biosafety is simply making sure that the GMOs are safe to the environment, biodiversity and human health. To commit towards ensuring the safe use of GMOs, Sri Lanka ratified the CPB in 2004.
The adherence to the CPB enables reaping the benefits of modern biotechnology with assurance that GMOs are not harmful to the environment, biodiversity and human health. Recognizing the need for adequate levels of protection in the safe use of modern biotechnology, the BDS formulated the National Biosafety Framework (NBF) and the National Policy on Biosafety. Both of these documents were approved by the cabinet of ministers in 2005. Additionally, the BDS worked with the Legal Draftsman’s Department to prepare the draft Biosafety Act, a specific law to deal with GMOs in Sri Lanka.
Nevertheless, implementation of the NBF requires sufficient capacity in many aspects including regulations, risk assessment, detection and awareness on the products of modern biotechnology. Therefore, there was an urgent need to build Sri Lanka’s capacity to make greater use of the benefits of GMOs in a safe and sustainable manner.
Understanding this requirement of building the capacity of Sri Lanka to establish biosafety in the country, the national focal point for biosafety, MoE, partnered with the Food and Agriculture Organization of the United Nations (FAO) for technical support and initiated the National Biosafety Project in 2017. This ongoing project is funded by the Global Environment Facility (GEF), an international funding entity helping to tackle our planet’s most pressing environmental problems.
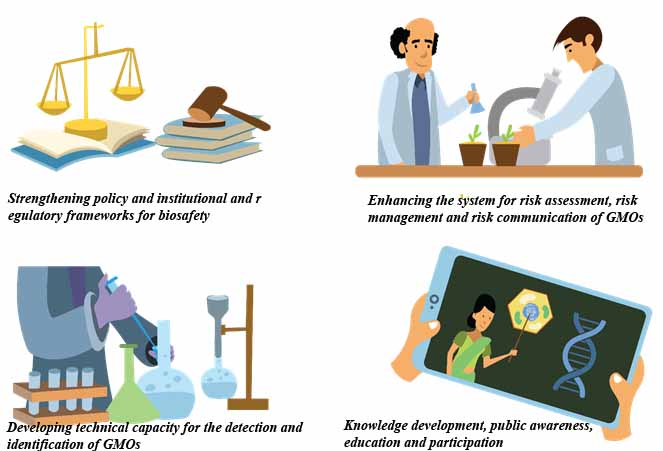
The objective of the Biosafety Project is to strengthen the regulatory, institutional and technical capacities for the effective implementation of the NBF in conformity with the CPB. This objective is being achieved through; Component 1 – strengthening policy, institutional and regulatory frameworks for biosafety, Component 2 – enhancing the system for risk assessment, risk management and risk communication of GMOs, Component 3 – developing technical capacity for the detection and identification of GMOs, and Component 4 – supporting targeted education and outreach campaigns to raise awareness about biosafety and enhancing public participation in decision-making.
The success of the project is a result of effective technical support provided by FAO through national and international consultants as well as several implementing partners. The Biotech Consortium India Limited (BCIL), New Delhi, India is technically supporting components 1, 2 and 3. The Agriculture Biotechnology Center (AgBC) of the University of Peradeniya is technically supporting component 3 as well as preparing curriculum and educational material on biosafety for secondary and tertiary level education. The National Science Foundation (NSF) is technically supporting component 2 and the development of awareness material for dissemination of knowledge in biosafety among several stakeholder groups. The project has achieved many milestones throughout the past few years in all four components of the project.
While it is essential to have an effective regulatory system to implement biosafety in the country it is also Sri Lanka’s national obligation as a signatory to the CPB. The key achievements under the project towards strengthening the regulatory system of biosafety in Sri Lanka are preparation of the draft Biosafety regulations, the Biosafety Master Plan and the draft Manual on Administrative and Operational Procedure for handling applications of GMOs.
We are depended on the internet all the time for information and this is true for biosafety as well. To fulfill the need for quick access of reliable information on biosafety of Sri Lanka, a dedicated website titled “Sri Lanka Biosafety Clearing House (BCH)” was established. The Sri Lanka BCH is the portal for all the information related to biosafety in the country, which is connected to the central portal of global BCH.
It is essential that Sri Lanka has the expertise to conduct risk assessment of GMOs in a scientifically sound manner. In order to strengthen this capacity in Sri Lanka, the project drafted the guidelines pertaining to risk assessment of GMOs. They are; (i) Guidelines for the safe use of GMOs in the lab, (ii) Guidelines for the environmental risk assessment of GM plants, (iii) Guidelines for the conduct of confined field trials of GM plants, (iv) Guidelines for the safety assessment of foods derived from GM plants, (v) Guidelines for the testing of GM mosquitoes, (vi) Guidelines for the institutional biosafety committees and (vii) The risk analysis framework. Further, the project is planning to train relevant individuals to conduct risk assessment of GMOs.
Although GMOs are different from their non-GMO counterparts at the molecular level, they appear similar to each other. Therefore, it is important that Sri Lanka has the capacity to detect and identify the GMOs using the necessary tools and techniques. This requires molecular testing laboratories with suitable equipment and human resources in the country. The project visited and assessed several national laboratories to check if they have the prerequisite infrastructure which could be upgraded to conduct regulatory testing of GMOs. The laboratories at the National Plant Quarantine Services, the Industrial Technology Institute (ITI) and the Agriculture Biotechnology Centre (AgBC), University of Peradeniya were selected to be upgraded for regulatory testing of GMOs.
Whether people prefer to use GMOs or to avoid them, it is a personal choice. However, misinformation and misconception about GMOs may lead to dire consequences in decision making. Therefore, it is important that the public is well aware about modern biotechnology, its products and biosafety. Especially when it comes to approval of a GMO in the country, it is crucial that people participate in informed decision-making. Thus, knowledge development and awareness on biosafety among all stakeholder groups including the public is essential towards successful implementation of the NBF in Sri Lanka.
To ensure that all relevant stakeholders have sufficient knowledge in modern biotechnology and biosafety to make informed decisions, awareness activities were initiated by the project. To enrich these activities with essential information, the project has developed several awareness material in all three local languages. Further, the project initiated the publication of a biannual and trilingual newsletter on biosafety. The attempt to make people aware about biosafety wouldn’t be complete if this subject is not integrated into the education system. Therefore, the project is developing curriculum and course material to integrate biosafety into secondary and tertiary education levels in Sri Lanka.
Although biosafety is just one aspect to consider when it comes to safeguarding biodiversity in Sri Lanka, the country is doing its best to ensure biosafety with the technical support from FAO through the National Biosafety Project. This way, the valuable biodiversity of Sri Lanka will not be threatened even if the country makes use of modern biotechnology if needed.



National laboratories were assessed for their suitability to be upgraded to conduct regulatory testing of GMOs in Sri Lanka – May 2019
(i) National Plant Quarantine Services (NPQS), (ii) Government Analysist’s Department, (iii) Sri Lanka Customs, (iv) Industrial Technology Institute (ITI), (v) Agricultural Biotechnology Centre (AgBC) – University of Peradeniya, and (vi) Institute of Biochemistry, Molecular Biology & Biotechnology (IBMBB).